The steady concentration of ozone in the stratosphere depends on the balancing processes of the formation and destruction of ozone.When ultraviolet light strikes a molecule,ozone is formed. In nature,this is a slow process with a regeneration half-time of three to four years. In the lower atmosphere,ozone is formed when oxides of nitrogen and hydrocarbons emitted from motor vehicles,power plants and other sources come together in presence of sunlight and water vapour. Ozone(O3) readily absorbs ultraviolet light and dissociates into O2 and o. The stratosphere is one of the middle layers of the atmosphere that starts some ten miles above the Earth’s surface and extends to a height of about 30 miles.
While ozone in the stratosphere is beneficial by protecting us,and also animals and plants from the sun’s ultraviolet rays,ozone in the lower atmosphere or troposphere is the chief component of smog which damages trees,plants and animals. Ozone will react with anything in sight including lung and eye tissues. Pollution of the lower atmosphere by ozone and carbon monoxide is a cause for concern.
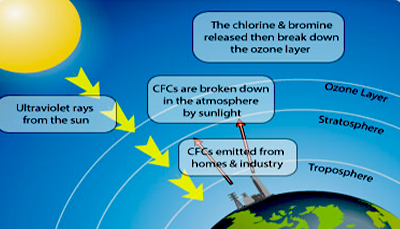
Stratospheric ozone is just as harmful to breathe,but nobody is up there to breathe it. In this region,ozone molecule created by solar radiation, form a protective blanket around the Earth and absorb most of the ultraviolet light from the sun,preventing it from reaching the Earth. Humans are thinning this blanket by releasing ozone- destroying chlorine and bromine atoms into the atmosphere. Small amounts of chlorine readily destroy very large quantities of ozone. Ozone has been found to be more depleted in the Antarctic than in the Arctic,referred to as the “ ozone hole”. Harmful ultraviolet rays passing through this hole can break up DNA molecules and increase the incidence of malignant melanomas,skin cancers and cataracts,and may also harm marine life and crops. Sensitive crops like soya beans are easily destroyed by the increase in ultraviolet radiation caused by depletion of the ozone layer in the upper stratosphere.
Chlorofluorocarbons (CFCs) are almost inactive at ground level which allows them to rise unchanged into the stratosphere. CFCs, used as propellants in aerosol spray cans, as foam blowing, as refrigerants in freezers and air-conditioning machines,as solvent in the electronic industry, and in inhalers being used daily by millions of asthmatic patients, release free chlorine in the presence of sunlight. This free chlorine deplete the ozone in the ozone layer present some 15 miles above the earth. CFCs in the aerosols contribute some 25% while medical aerosols inhalers only 0.5%. If fluorocarbons are allowed to increase every year, the ozone might be depleted by a substantial amount in a few decades again. This might be enough to destroy all life including mankind. Millions of tons of CFCs have already been realised into the environment. This has been a source of serious concern to the environmentalists.
The damage responsible for the ‘ozone hole’ has been blamed on chlorine driver not only from hydrocarbons but also from other chemicals such as carbon tetra chloride, methyl-chloroform and bromine contains halogens from fire extinguishers. Oxides of nitrogen emitted from engines of supersonic planes operating in the stratosphere, also react with the ozone. Bromide (used in the manufacture of plastics and fungicides for crops), nitrates (used as fertilisers) and chlorine (used for treating water and sewage) may also threaten the ozone layer. Bromine is so effective in depleting the ozone layer that if injected into the stratosphere over enemy territory, bromine could cause destruction of crops.